Whenever a cell's genetic material is damaged, a host of processes kick in, which eventually allow the cell to repair itself, and most importantly its DNA. Researchers have now discovered the intricacies of this process, as well as its most important components.
The key piece in this complex puzzle is a molecular motor that investigators determined to be extremely flexible. This ability goes a long way towards ensuring that our bodies don't need to waste resources replacing every single cell that gets even slightly damaged.
This study was conducted by scientists at the US Department of Energy’s (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab), in collaboration with colleagues from the Scripps Research Institute (SRI).
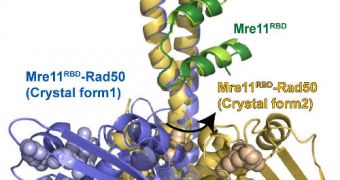
Past studies had already demonstrated that a DNA repair machine called MRN plays an important role in these repairs. What the team did was study both of its molecular components, and determine the way they interact in order to underly this ability.
For this purpose, the team used Berkeley Lab's Advanced Light Source (ALS) synchrotron. This particle accelerator is capable of producing high-energy X-rays, which are commonly used to study the inner working of molecules, and the fundamental properties of substances.
What surprised the experts was one of the two MRN components, called Rad50. This molecular motor was found to be incredibly flexible in its natural state, when the cell did not call upon it for repairs.
However, when the signals were received, the motor clamped shut like pliers. This happened immediately after it got connected to ATP (adenosine triphosphate), the energy molecules cells in general operate on. ATP is produced in structures called mitochondria.
“We had no idea this motor is tethered to the repair machine in such a flexible way,” explains researcher John Tainer. He holds joint appointments at the Berkeley Lab Life Sciences Division and the SRI, in La Jolla, California.
The expert is a co-leader of the investigation, together with SRI colleague Paul Russell. Details of the study were published in the March 27 advance online edition of the scientific journal Nature Structural and Molecular Biology.
“This superfamily motor is so versatile because it’s flexibly tethered. Before this research, we believed it only went from open to closed. But now we know it can be open, closed, and anywhere in between,” Tainer concludes.
Funding for this research came from the National Cancer Institute and the National Institutes of Health (NIH) Intramural Research program. Additional funds came from the DOE Office of Science.
Video Description: Rad50’s ability to morph between multiple shapes is essential for the function of MRN in DNA repair. Video Credit: Berkeley Lab
 14 DAY TRIAL //
14 DAY TRIAL //