University of Toronto chemistry professor Greg Scholes strongly believes that we can improve our renewable energy production methods by a wide margin, if only we could learn to emulate the highly-successful designs that can be met anywhere in nature.
He is mostly referring to solar energy, as this is the vastest source of energy at our disposal. Despite years of work and billions of dollars, scientists have yet to develop solar cells capable of harnessing more than 18 percent of the energy light hitting them carries.
But even the most underdeveloped plant capable of conducting photosynthesis is several times more efficient in absorbing both heat and light. Learning how to emulate this process would literally put us millions of years ahead of the curb – the time it took nature to develop it from scratch.
At this point, the fact that our species will not be able to survive in the long-run without the aid of clean energy solutions is becoming increasingly clear. Therefore, we must learn to capture, transfer and store solar energy in a manner that is cost-effective, efficient, and that prevents unnecessary loss.
Scholes, who is the D.J. LeRoy Distinguished Professor at the University of Toronto, detailed his concepts for the future in a paper published in the September 23 issue of the top scientific journal Nature Chemistry.
Experts from the University of California in Berkeley (UCB), the University College London (UCL), and the VU University, in Amsterdam, also contributed on the work. The paper is entitled “Lessons from nature about solar light harvesting.”
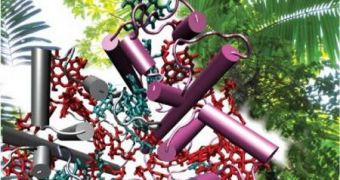
“Solar fuel production often starts with the energy from light being absorbed by an assembly of molecules. The energy is stored fleetingly as vibrating electrons and then transferred to a suitable reactor,” the team leader explains.
“It is the same in biological systems. In photosynthesis, for example, antenna complexes comprised of chlorophyll capture sunlight and direct the energy to special proteins called reaction centers that help make oxygen and sugars. It is like plugging those proteins into a solar power socket,” he goes on to say.
The team highlights the fact that one of the key aspects of photosynthesis – and one that is likely to prove very difficult to emulate – is the fact that dye molecules inside leafs move so fast that they simply do not allow the sunlight energy they store time to dissipate and get lost.
“This is why natural photosynthesis is so inspiring. More than 10 million billion photons of light strike a leaf each second. Of these, almost every red-colored photon is captured by chlorophyll pigments which feed plant growth,” Scholes concludes.
 14 DAY TRIAL //
14 DAY TRIAL //