Scientists have identified a malformed protein common to two deadly neurological diseases, frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig's disease).
"This exciting basic science discovery provides the first molecular link between a dementia--FTD--and a motor neuron disease--ALS. It will advance understanding of the pathological processes of FTD and ALS, and possibly of other neurological disorders," says NIA director Richard J. Hodes, M.D.
"Improved understanding of underlying disease processes is critically important in pointing researchers toward the development of therapies for FTD, ALS and other neurodegenerative diseases," Hodes notes.
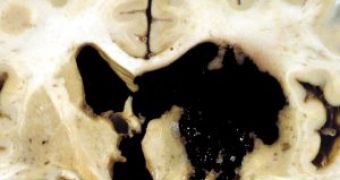
FTD provoke the frontal and temporal lobes of the brain to shrink (image) and also lead to certain behavioral changes. They may exhibit uninhibited and socially inappropriate behavior, becoming fixated on sex or engage in criminal behavior, and, in late stages, loss of memory, motor skills, and speech. This is the second type of dementia in people under age 65 after Alzheimer's disease. ALS causes progressive degeneration in motor neurons. In time, walking, eating, speaking and breathing become more difficult in this fatal disease.
Some people with ALS also have FTD, and some with FTD also develop ALS, suggesting that they might have shared a common pathway to cell death. In some neurodegenerative diseases - including ALS and FTD - scientists have identified malformed proteins that accumulate in nerve cells.
Neurobiologists Virginia Lee, Ph.D., and John Trojanowski, M.D., Ph.D., of the University of Pennsylvania have long sought to solve why they form and what they contain. They have now identified TDP-43 as a constituent part of the clumps that form in ALS and in FTD. In about half of FTD cases, brains are speckled with protein balls containing tau, a protein implicated in Alzheimer's and other brain diseases.
In the other fraction of FTD cases, the deposited protein was unknown. To identify the molecule, the researchers isolated a group of proteins from brain samples of people with FTD. They injected the molecules into mice to create antibodies capable of recognizing the various suspect proteins. Searching through the antibodies to find those that stuck only to the balls in brain samples of FTD patients, they found out an obscure protein, TDP-43.
The same antibodies gathered to malformed protein in motor neuron samples from ALS patients, indicating that was the same protein, TDP-43. Till now, it is known that TDP-43 takes part in transcribing and regulating genetic information in the nucleus of the cell. "There is much more to learn about how this nuclear protein is clumped in the cytoplasm of cells and about the mechanism by which it is implicated in two distinctly different diseases," says Stephen Snyder, Ph.D., program director, etiology of Alzheimer's disease, NIA Neuroscience and Neuropsychology of Aging Program.
"It is possible that the TDP-43 protein will be a key to a more complete understanding of both FTD and ALS."
"What that strongly suggests," says neurobiologist Michael Hutton of the Mayo Clinic in Jacksonville, Florida, "is that in each of these diseases there is at some point a common pathway to neuronal cell death. We've long suspected this might be the case; we just didn't realize it was the same major protein accumulating."
 14 DAY TRIAL //
14 DAY TRIAL //