Clinicians are about to receive the first new set of diagnostics criteria for Alzheimer's disease in nearly three decades. The last revision of these criteria took place some 27 years ago. The new modification was made so that research, diagnosis and therapies reflect the latest discoveries.
This revision, which was funded by the US National Institutes of Health (NIH), also proposes new ways of separating this neurodegenerative condition into stages, and of using biomarkers to gain more information about how it is progressing in the human brain.
The new report is called the “National Institute on Aging/Alzheimer's Association Diagnostic Guidelines for Alzheimer's Disease.” The two organizations led the effort to centralize, analyze and include new data into this important set of guidelines.
“Alzheimer's research has greatly evolved over the past quarter of a century. Bringing the diagnostic guidelines up to speed with those advances is both a necessary and rewarding effort that will benefit patients and accelerate the pace of research,” NIA Director Richard J. Hodes, MD, explains.
The previous version of this document only described and addressed later stages of Alzheimer's development, when symptoms usually associated with dementia were already obvious. Emphasis is nowadays placed on early detection, rather than on aggressive treatment.
Updated guidelines made available in the new document now cover all aspects of the disease's progression, ranging from when symptoms are not yet obvious to full-blown cases of dementia.
Scientists also included new data about the earliest, pre-clinical stages of the condition, about mild cognitive impairment (MCI), which is an associated condition and an intermediary step to Alzheimer's and about the pathology of dementia that ensues with this disease.
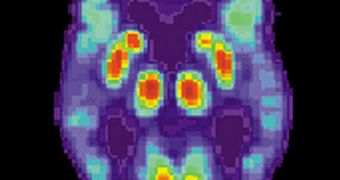
New information has also been made available about how to use biomarkers and various medical imaging technologies to observe the condition setting in the bloodstream and spinal fluid of patients.
“We believe that the publication of these articles is a major milestone for the field,” explains the chief medical and scientific officer at the Alzheimer's Association, William Thies, PhD.
“Our vision is that this process will result in improved diagnosis and treatment of Alzheimer's, and will drive research that ultimately will enable us to detect and treat the disease earlier and more effectively,” the official goes on to say.
“This would allow more people to live full, rich lives without – or with a minimum of – Alzheimer's symptoms,” Thies adds, saying that the new guidelines also include something that the previous set did not, which is flexibility.
This will allow for the seamless integration of new data and technologies in the existing dataset.
 14 DAY TRIAL //
14 DAY TRIAL //