According to experts in chemistry, diamond may in fact have a cousin. They say that T-carbon may be a form of the common chemical that is a bit softer than diamonds, but still harsher than other forms of the stuff, such as for example graphite.
Diamond is the most stable and organized form of carbon. It is only produced at tremendous pressures and temperatures, such as for example those found beneath Earth's crust, where tectonic plates reenter the mantle to get recycled.
At this point, experts know several allotrops (forms of) carbon, including diamonds, graphite, single-walled carbon nanotubes, amorphous carbon, buckminsterfullerene (C60) and lonsdaleite.
But, while these forms of the element have already been obtained or observed, there are still those that are only proposed. A team of Chinese experts has recently added yet another entry to the list of allotrops that needs to be verified.
A supercomputer revealed that a new form of carbon is possible, and the team called it T-carbon. The material has a very organized structure as well, but the science group has yet to demonstrate that it can be produced in reality, or that it remains stable in time.
“What is the most surprising to us is that such an elegant structure has never been proposed before,” explains scientist Gang Su. He holds an appointment as a materials scientist at the Graduate University of the Chinese Academy of Sciences (CAS), in Beijing.
The expert is also a coauthor of a new paper describing T-carbon, which is scheduled to be published in an upcoming issue of the esteemed scientific journal Physical Review Letters, Science News reports.
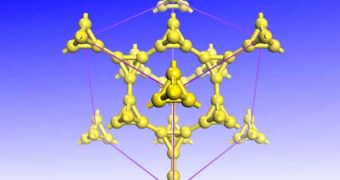
“It would be a very light, hard material, so you could imagine a large number of applications,” explains Stanford University geophysicist Wendy Mao, who was not part of the new study. Su got the idea about the new form of carbon after seeing the pyramids of Giza. The expert realized that the crystalline structure of diamonds could be modified by replacing each of the carbon atoms with a tetrahedron made up of 4 carbon atoms.
The resulting carbon allotrop would have 43 percent the density of diamonds, and 65 percent of their hardness. Simulations of the flow of electrons through the new material prove that it could be used as a semiconductor as well.
But not all experts in the field are convinced that T-carbon could ever be created in a stable configuration.
“Even if it was synthesized, I don’t know if it would hold together. The energy of this stuff is much, much higher than other forms of carbon, and little perturbations might cause the structure to collapse,” says University of Minnesota in Minneapolis materials scientist Renata Wentzcovitch.
 14 DAY TRIAL //
14 DAY TRIAL //