A team of investigators from the University of Cambridge has recently discovered that a simple chemical reaction can make all the difference in determining how carbon dioxide spreads through underground aquifers.
Geologists define an aquifer as a layer of permeable rock or unconsolidated material that contains water, and which can surrender this water for human use if a well is dug. Groundwater can be found in gravel, silt and sand too.
An issue affecting the purity of groundwater in aquifers is the fact that carbon dioxide can easily spread through the water under some conditions. But this spread can be delayed, or even stopped completely, the Cambridge team has shown.
The research group demonstrated that the distinct scenarios of CO2 transport that exist in saline underground water reserves are directly linked to the strength of the chemical reaction that develops between the carbon dioxide and the porous rock containing the water.
“If one knows the physical properties of the aquifer, one can now calculate the movement of CO2 across it, and when it will begin to mix with the brine,” explains research scientist Jeanne Andres.
“In theory, one can manipulate the strength of reactions, thereby engineering the movement of CO2 – keeping it in one area or moving it to another within the aquifer – to enhance its storage underground,” the expert explains.
Andres holds an appointment as a Schlumberger Foundation PhD researcher in the Cambridge Department of Chemical Engineering and Biotechnology. She explains that the new work has considerable implications for carbon sequestration technologies (CST).
CST are currently being investigated as one of the most promising methods of preventing CO2 from making its way into Earth's atmosphere and boosting the global warming effect that is now driving changes in Earth's climate.
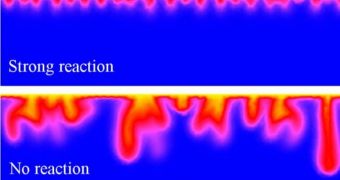
The team demonstrated that a weak reaction between CO2 and the porous rocks in the aquifer will allow the chemical to spread down to the deepest layers of the underground structure. Conversely, a stronger reaction will confine the CO2 to the upper layers of the aquifer.
Details of the new work appear in a paper entitled “Onset of convection in a porous medium in the presence of chemical reaction,” which was published in a recent issue of the esteemed scientific journal Physical Review E.
“This research shows how rigorous mathematical analysis coupled with strong physical understanding can help us grasp the complex interactions of flow and reaction in a carbon reservoir,” comments Dr. Silvana Cardoso.
“Such knowledge will be valuable in guiding future approaches to carbon storage,” she adds. Cardoso is the leader of the research project, and she is a Reader in the Cambridge Department of Chemical Engineering and Biotechnology.
 14 DAY TRIAL //
14 DAY TRIAL //