Carbon nanotubes (CNT) are among the most promising new materials around at this point. They exhibit amazing chemical and physical properties, and could be of tremendous use in future, electronic devices. A large number of research groups is looking into customizing their properties, as it became obvious that they could be grown to have either metallic or semiconducting properties long ago. Now, a team of scientists at the Purdue University has managed to finally establish a thorough, scientific process for making that a reality, and designing custom CNT.
“This problem of how to control whether you have a metal or a semiconductor is the key stumbling block in making transistors out of carbon nanotubes. Solid-state electronics is built around the fact that you can control the semiconducting properties of silicon,” PU Associate Professor of Materials Engineering Eric Stach explains. When properly manufactured, the carbon nanotubes are able to conduct electricity better than any other kind of metal. Experts from the Honda Research Institute USA Inc. Materials Science Division and the University of Louisville have also contributed to the research.
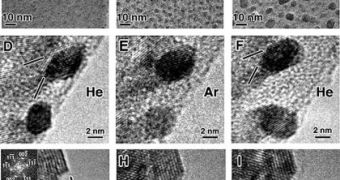
“This is the first report that shows we can control pretty systematically whether carbon nanotubes are metallic or semiconducting. We have a 91 percent success rate of producing metallic nanotubes,” HRI principal scientist Avetik Harutyunyan, who has also been the leader of the study, adds. Details of the method appear in the October 2nd issue of the top journal Science. “Generally, carbon is not a metal, but carbon nanotubes with a particular configuration are,” Stach adds. The team determined that the simplest way to create whichever kind of CNT it pleased was by adding argon or helium gas to the deposition chambers, where regular nanotubes were formed.
The Purdue scientists were able to confirm that tweaking the production process in this manner resulted in the creation of either metallic or semiconducting nanotubes. They also provided photographic evidence of this, when they imaged the CNT using a transmission electron microscope. The University of Louisville was able to take extremely precise measurements of the tubes, and determined the cases in which they were either metallic or semconducting, and measured each instance's electrical properties.
“These findings provide a window into the intimate relationship between the atomic structure of the catalyst nanoparticle and the carbon nanotube that grows from that catalyst nanoparticle. The results also show that the atomic structure of the catalyst nanoparticle can be controlled by the ambient carrier gas, a linkage that may represent the first step toward a solution to one of the most vexing challenges in nanotechnology,” the Mary Jo and Robert L. Kirk Director of the Birck Nanotechnology Center, Timothy D. Sands, concludes.
 14 DAY TRIAL //
14 DAY TRIAL //