A new technology could soon revolutionize health care, and highly improve patients' chances of recovery and survival, by diagnosing them in real time, after a simple analysis of the biomarkers (chemical compounds) in their breath.
This technology detects changes in electrical resistance or conductance, as gasses pass over sensors placed on top of 'micro-hotplates' – small heating devices on electronic chips.
Detecting these biomarkers can give many information about the patient's health state, whether he/she is suffering from cancer or other diseases.
Carlos Martinez, an assistant professor of materials engineering at Purdue University, working with researchers at the National Institute of Standards and Technology, said that “we are talking about creating an inexpensive, rapid way of collecting diagnostic information about a patient.
“It might say, 'there is a certain percentage that you are metabolizing a specific compound indicative of this type of cancer,' and then additional, more complex tests could be conducted to confirm the diagnosis.”
For now, the scientists used the technology to detect acetone, which is a biomarker for diabetes, and they reached a sensitivity in the parts per billion range in a gas imitating a person's breath.
With this, they proved that their method can detect biomarkers at least 100 times better than previous technologies focusing on breath.
“People have been working in this area for about 30 years but have not been able to detect low enough concentrations in real time,” Martinez said.
"We solved that problem with the materials we developed, and we are now focusing on how to be very specific, how to distinguish particular biomarkers."
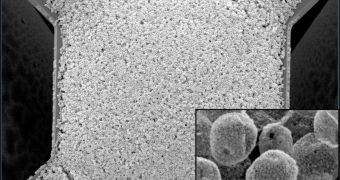
Their approach consisted in using a template made of micron-size polymer particles and coating them with far smaller metal oxide nanoparticles.
On each micro-hotplate (of about 100 microns square and containing electrodes shaped like meshing fingers), a droplet of the nanoparticle-coated polymer microparticles was placed.
Once the droplet dried, the electrodes were heated up, burned off the polymer and left a porous metal-oxide film, creating a sensor.
The nanoparticle-coated microparticles are better than flat surfaces because they increase the porosity of the sensor films, improving the sensitivity of the 'active sensing surface area'.
“It's very porous and very sensitive,” said Martinez and “we showed that this can work in real time, using a simulated breath into the device.”
However, this type of breathalyzers are not going to be available tomorrow, nor for the next decade, mainly because nobody has yet developed precise standards that would allow manufacturing the device based on this approach.
“However, the fact that we were able to do this in real time is a big step in the right direction,” added Martinez.
These findings were detailed in a research paper that appeared earlier this year in the IEEE Sensors Journal, published by the Institute of Electrical and Electronics Engineers' IEEE Sensors Council.
 14 DAY TRIAL //
14 DAY TRIAL //