US Department of Energy (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab) physicists announce the development of a new approach to improving lithium-ion batteries, the most common portable energy-storing devices in use today.
While these batteries provide acceptable performances for cell phones, laptops, tablets and mp3 players, they are still not sufficiently developed for use in more complex applications, such as for example electric cars capable of covering a lot of ground on a single charge.
Experts know that these designs could be improved considerably, and they also know how this objective can be achieved. What they don't know is how to get to that level of development which allows them to extend the range of uses Li-Ion batteries have.
Berkeley Lab experts recently took a step in this direction, when they created an anode for lithium-ion batteries that can store 8 times more lithium than existing anodes. This greatly increased energy capacity was maintained ever after hundreds of charge-recharge cycles.
The team explains that they tested the new anode design for about a year, before deciding to publish their work. Their paper, “Polymers with Tailored Electronic Structure for High Capacity Lithium Battery Electrodes,” appears in the latest online issue of the scientific journal Advanced Materials.
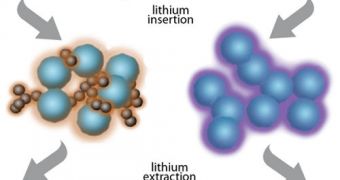
According to the scientists, the key to the new anode's capabilities is a special type of polymer that is capable of conducting electricity with extreme efficiency. The new material can also bound closely to lithium-storing silicon particles.
During the charging cycle, the polymer expands three times from its original volume, and deflate by the same amount while the lithium ions are released towards the cathode. Furthermore, the innovative material is made out of cheap, readily-available components.
The polymers can easily be manufactured using existing production lines, without the need to change the technological process. “High-capacity lithium-ion anode materials have always confronted the challenge of volume change – swelling – when electrodes absorb lithium,” Gao Liu explains.
The expert, who is based at the Berkeley Lab Environmental Energy Technologies Division (EETD), is a member of the Batteries for Advanced Transportation Technologies (BATT) program. The latter is supported by the DOE Office of Vehicle Technologies.
“Most of today’s lithium-ion batteries have anodes made of graphite, which is electrically conducting and expands only modestly when housing the ions between its graphene layers,” Liu adds.
“Silicon can store 10 times more – it has by far the highest capacity among lithium-ion storage materials – but it swells to more than three times its volume when fully charged,” he concludes.
 14 DAY TRIAL //
14 DAY TRIAL //