A report published in yesterday's issue of the journal Science Express details how, using an X-ray laser, scientists managed to observe atoms snuggling up to each other and birthing new molecules.
The experiments, carried out by brainiacs with the SLAC National Accelerator Laboratory in the US, are expected to pave the way for a better understanding of how chemical reactions play out.
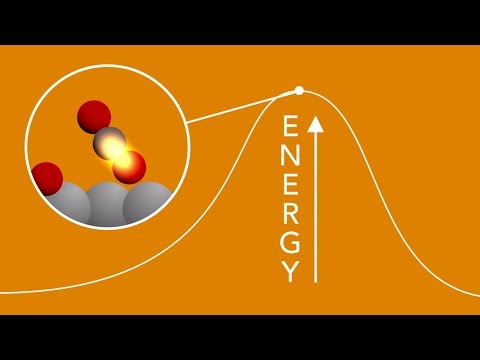
As detailed in the video below, the researchers behind this investigation started by attaching carbon monoxide (CO) and oxygen (O) atoms to the surface of a catalyst. They then fired an optical laser at them.
The pulse originating from this optical laser caused the catalyst to heat up. When this happened, the CO and the O began to vibrate. Eventually, the atoms started bumping into one another and connecting.
With the help of X-ray laser pulses, the SLAC National Accelerator Laboratory researchers managed to observe the tentative bonds that almost instantly formed between the atoms that they toyed with.
“First the oxygen atoms get activated, and a little later the carbon monoxide gets activated,” specialist Anders Nilsson explained in a statement.
“They start to vibrate, move around a little bit. Then, after about a trillionth of a second, they start to collide and form these transition states,” he added.
What's interesting is that, of the atoms that grew quite fond of each other, not all made the shift from tentative bonds to proper molecules. On the contrary, plenty simply grew estranged again after a while.
Specialists explain that, the more is known about how chemical reactions take place, the easier it will be to develop new and more efficient ways to generate energy, even improve fertilizers and day-to-day products.

 14 DAY TRIAL //
14 DAY TRIAL //