Purifying river and lake water for human consumption is a very dangerous and tricky business, and one that does not leave the smallest room for errors. If dangerous bacteria, microbes or other microorganisms are let through, thousands of people get sick in a few days, causing outbreaks of various diseases, which are then carried throughout countries by tourists and businessmen. To alienate these concerns, experts at the Sandia National Laboratories (SNL) have improved the basic decontaminant used for these waters, by simply removing an atom from a molecule found in it.
Although this may not seem like much, the researchers say that this betterment in the production process guarantees an improved efficiency in the solution's action, as well as an extended shelf life, which was a problem until now. The stuff is able to clear water of both organic and inorganic trespassers, as well as of bacteria and microbes. Additionally, it can also be used to clean waste water in treatment plants, before allowing it to reenter nature's natural circuit, ScienceDaily informs.
“Human consumption of ‘challenged’ water is increasing worldwide as preferred supplies become more scarce. Technological advances like this may help solve problems faced by water treatment facilities in both developed and developing countries,” May Nymann, the principal investigator for the new research at the Sandia laboratories, explains. Details of the new process appear in the June issue of the American Chemical Society's publication Environmental Science & Technology.
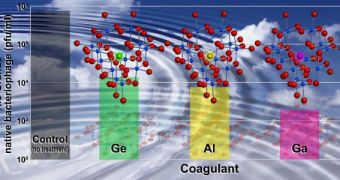
The basic substance of the research is the common water coagulant aluminum oxide, a cluster-shaped material. The SNL experts substitute a gallium atom from the cluster, by using a relatively simple set of chemical reactions. In the first stage, aluminum salts are dissolved in water, while gallium salts are mixed into a sodium hydroxide solution. The second step involves combining the two liquids slowly, while mixing and heating.
“The substitution of a single gallium atom in that compound makes a big difference. It greatly improves the stability and effectiveness of the reagent. We’ve done side-by-side tests with a variety of commercially available products. For almost every case, ours performs best under a wide range of conditions,” Nymann concludes.
 14 DAY TRIAL //
14 DAY TRIAL //