An international team of researchers coming from universities in the US, the UK and the Netherlands, recorded footage of atomic orientation undergoing amazing levels of control.
Claire Vallance of the University of Oxford, UK, along with David Parker at Radboud University Nijmegen, the Netherlands, and Richard Zare at Stanford University, US, focused on the atomic angular momentum vector precessing in a magnetic field, and to do so, they used velocity map imaging (VMI).
This technique is normally used to study gas-phase photochemical reactions, so the researchers used it to image molecular oxygen dissociated by a UV laser.
The oxygen that comes out of the reaction has a very high aligned orbital angular momenta, so when the scientists applied a magnetic field for a fixed time, they caused it to precess.
Next step was to ionize the atoms with another, polarized laser, and to use an electric field that would accelerate the ions towards a detector, which was able to give their positions in 3D.
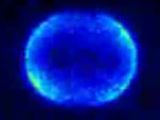
Vallance explained that “the movie shows three different slices through the 3D distribution.”
The quantum mechanical interaction between an atom's angular momentum distribution and the polarization of the laser, is what determines its ionization probability, thus its detection probability.
When applying the magnetic field for different durations, the researchers obtained time-lapse movies of the precessional motion.
“As you make the distribution precess, the detection probability of the atoms changes, and that's what you're actually seeing,” said Vallance.
This is the first time that someone has obtained images of an atomic angular momentum vector precessing in a magnetic field – which is a motion that looks like that of a spinning top coiling about Earth's gravitational field as it slows.
And since the angular momentum vectors have an associated magnetic moment that can be aligned with a magnetic field, precession rises – this is actually exploited in NMR spectroscopy and MRI.
The next step for Vallance and her colleagues is to study the way that orbital positioning influences chemical reactions, Chemistry World reports.
“In principle, we could take a p-orbital in an atom and see how its reactivity changes as it is rotated relative to a second reactant molecule.”

 14 DAY TRIAL //
14 DAY TRIAL //