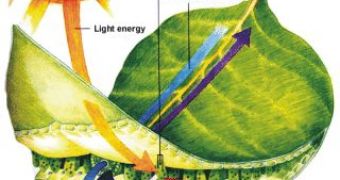
This is the process that fuels life on Earth: plants simply mix carbon dioxide from the air, water and sunlight to turn them into biomass and oxygen.
Chemists would also like to be able to use CO2 as a carbon source just like in the photosynthesis for synthesizing organic reactions, but this is not as simple as it might appear.
Researchers led by Markus Antonietti at the Max Planck Institute for Colloids and Interfaces have now made a step ahead toward imitating this.
By employing a special novel class of metal-free catalyst, graphitic carbon nitride, they succeeded to activate CO2 in a similar fashion.
"Chemical activation of carbon dioxide, meaning its cleavage in a chemical reaction is one of the biggest challenges in synthetic chemistry." explained Antonietti.
The carbon-oxygen bond in CO2 molecule is extremely stable, requiring a lot of energy to break and very few special metal catalysts can do it.
Unlike in previous researches, this time, the scientists used metal-free catalysts, more like what occurs in plants.
A crucial step in photosynthesis is the bonding of CO2 to nitrogen atoms to make carbamates.
The nitrogen-rich graphitic carbon nitride catalyst (made of flat, graphite-like layers made of rings of carbon and nitrogen atoms) allowed the carbamate synthesis .
The porous graphitic carbon nitride is also very heat-stable and, even if it participates to many chemical reactions, it maintains its stability, returning almost always to its initial shape, an ideal catalyst.
The carbon nitride oxidized benzene (an aromatic six carbon atoms ring) binds with phenol (which presents an extra OH group).
The by-product of this process is carbon monoxide (CO), directly implicated in photosynthesis.
Thus, CO2 was split into an oxygen atom and CO but in a photosynthesis carbamate fashion: CO2 has bound to free amino groups (containing nitrogen) present in the carbon nitride.
"This could make novel, previously unknown chemistry of CO2 accessible. It may even be the first step in artificial photosynthesis," said Antonietti.
 14 DAY TRIAL //
14 DAY TRIAL //