Engineering living nerves thus far has seemed next to impossible to most research teams, as evidenced by the little progress recorded in this field. However, it would seem that University of Pennsylvania School of Medicine (UPSM) scientists have manged to actually engineer brain nerves that can be transplanted in living animal models and facilitate the natural healing process of the organism.
“We have created a three-dimensional neural network, a living conduit in culture, which can be transplanted en masse to an injury site,” Penn Center for Brain Injury and Repair director Douglas H. Smith, MD, who is also a professor in the Department of Neurosurgery, shares. The expert is, furthermore, the senior author of a new paper detailing the find, published in the March issue of the journal Tissue Engineering.
In truth, Smith and his colleagues have created nothing short of several “jumper cables,” which act as bridges between the damaged sections of a nerve. These formations are made from axon bundles, engineered directly in the lab, which are transplanted into the area that needs repair, after they grow, and then help the host tissue regenerate itself.
“That creates what we call a 'nervous-tissue construct.' We have designed a cylinder that looks similar to the longitudinal arrangement of the nerve axon bundles before it was damaged. The long bundles of axons span two populations of neurons, and these neurons can have axons growing in two directions – toward each other and into the host tissue at each side,” Smith adds.
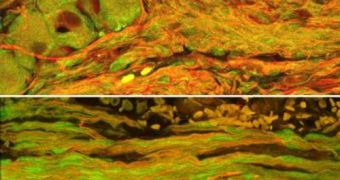
For the testing stage of the study, rats were used. A small portion of their sciatic nerves was removed, and then the creatures underwent surgery to have the lab-grown axon bundles placed inside them. Some 16 weeks after the operation, the area that had been operated showed unexpected signs of improvement, and the researchers noted that the transplanted axon bundles and the damaged ones seemed to have intertwined, creating a new form of axonal regeneration.
“Regenerating axons grew across the transplant bridge and became totally intertwined with the transplanted axons,” Smith underlines. In addition, the graft neurons were found to have their extremities deeply rooted within the host ones, which meant that they had actually survived the procedure, even though no immunosuppressive drugs had been used.
“This may be a new way to promote nerve regeneration where it may not have been possible before. It's a race against time – if nerve regeneration happens too slowly, as may be the case for major injuries, the support cells in the extremities can degenerate, blunting complete repair. Because our living axonal constructs actually grow into the host nerve sheath, they may 'babysit' these support cells to give the host more time to regenerate,” post doctoral fellow D. Kacy Cullen, PhD, the co-first author of the paper, concludes.
 14 DAY TRIAL //
14 DAY TRIAL //