Most electronics nowadays, portable ones, use lithium-ion batteries, and there are some that actually last for hours or days, even weeks depending on how small a device is and how little energy is required.
And yet the charge capacity of these batteries is no longer considered satisfactory, at least by scientists from Stanford University.
Then again, research is being done all the time, which means that a newer and better alternative to li-ion batteries would have been invented eventually even if there hadn't been incentive to do so.
Long story short, Stanford University scientists have invented an advanced zinc-air battery with higher catalytic activity and durability.
"With ample supply of oxygen from the atmosphere, metal-air batteries have drastically higher theoretical energy density than either traditional aqueous batteries or lithium-ion batteries," said Hongjie Dai, a professor chemistry at Stanford and lead author of the study.
"Among them, zinc-air is technically and economically the most viable option," he said.
Dai finds it curious that so much attention has been invested in li-ion batteries despite their limited energy density, safety problems and high cost.
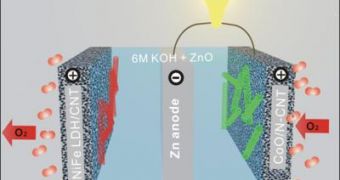
Zinc-air batteries produce electricity by combining atmospheric oxygen and zinc metal in a liquid alkaline electrolyte. Zinc oxide is a byproduct, but recharging reverses the process and regenerates oxygen and zinc metal.
The example in the photo enclosed up on the left is of a rechargeable zinc-oxide battery in a tri-electrode configuration. The nanotube is made of cobalt-oxide/carbon, while the catalysts for charge and discharge are composed of iron-nickel/layered double hydroxide.
"There have been increasing demands for high-performance, inexpensive and safe batteries for portable electronics, electric vehicles and other energy storage applications. Metal-air batteries offer a possible low-cost solution," said Hongjie Dai.
"Zinc-air batteries are attractive because of the abundance and low cost of zinc metal, as well as the non-flammable nature of aqueous electrolytes, which make the batteries inherently safe to operate. Primary (non-rechargeable) zinc-air batteries have been commercialized for medical and telecommunication applications with limited power density."
 14 DAY TRIAL //
14 DAY TRIAL //