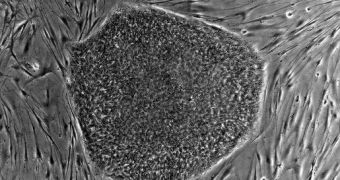
For the first time in more than ten years, scientists in the United States will finally have access to embryonic stem cells. These cells, which are the first ones to divide inside a new organism, have the ability to differentiate into any type of other cells in the body easily, and, as such, hold great promise for treating a large number of medical conditions, ranging from blindness to paralysis and cancer. But the US regulators' decision, of releasing just 13 varieties for research, already comes too late. The US has lost its leading role many years ago, in front of countries such as Japan, which now leads the global investigations in stem-cell research, the BBC News reports.
The new move was only made possible by the fact that US President Barack Obama stuck to his word and eased some of the regulations the Bush regime set in place while it ruled the country. Some analysts say that 96 more lines could be approved for scientific purposes soon, if they are found to meet the ethical guidelines that were adopted this July. “I am happy to say that we now have human embryonic stem cell lines eligible for use by our research community under our new stem cell policy,” the Director of the US National Institutes of Health (NIH), Francis Collins, says. One of the main reasons why these lines were released is the tremendous promise that they hold for curing diseases.
There are other stem-cell lines that have the same ability. For example, induced pluripotent stem cells (iPS) are adult cells that can be made to revert to their original settings, and act like stem cells. However, this process is very complex and time-consuming, and clear methods of using it at a large scale have yet to be set in place. The main advantage that the embryonic cells have is that they are ready to be used as soon as they are harvested, experts say. In addition, the latter type can also withstand indefinite replication inside labs.
The 13 new lines were created with private funds, and only relied on embryos collected from fertility clinics, and having full parental consent to do so. This was entirely done by the book, according to the July regulations, analysts say. The new rules appeared in order to allow researchers to start work on stem-cell lines that the former administration blocked for more than eight years, causing damage to the American scientific community, and sick people around the world, which may have benefited from new treatments, made possible by stem-cell research.
 14 DAY TRIAL //
14 DAY TRIAL //